Lithium-ion batteries are one of the most popular types of rechargeable batteries found in consumer electronics. In such batteries, lithium ions move from the negative electrode to the positive electrode when discharging; and return when being charged. To achieve this, these electrodes are constructed out of an intercalated lithium crystallite (a compound that allows for the reversible inclusion of lithium atoms).
In our research, we are interested in mechanical phenomena that arise in the crystallite due to the addition or removal of lithium atoms. This is especially interesting to study near regions of sharp interfaces between crystallite with and without lithium atoms, as the lack of coherency across the interface can cause the material to strain or even crack.
By modelling the intercalation process through the evolution of a thermodynamic potential and allowing for different mechanical properties of the two phases, we can study the evolution of multiple phases:
Time-dependent phase separation into Li-rich and Li-poor phases.
as well as the mechanical strains this phase transfer induces:
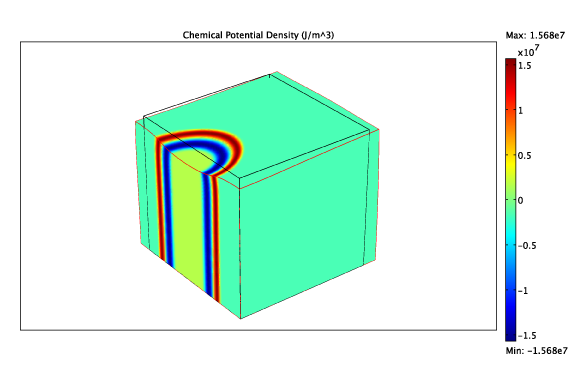
The chemical potential and deformation due to a LiFePO4 finger.