Although osteoarthritis is the most commonly occurring joint disorder, there exist no drugs that slow down the progression of the disease. Sufferers of the condition are thus subject to painful and reduced mobility, resulting in lowered quality of life.
This debilitating joint disorder is generally accepted to be caused when cartilage between the bones starts to wear out. Cartilage is the soft tissue that cushions bones at joints and usually allows for the bones to slide past each other. We are therefore interested in better understanding wearing out of this tissue layer, but more importantly, the biological processes involved in its formation, so worn out tissue can be replaced. For this, we turn our attention to the cell responsible for the synthesis of cartilage: the chondrocyte.
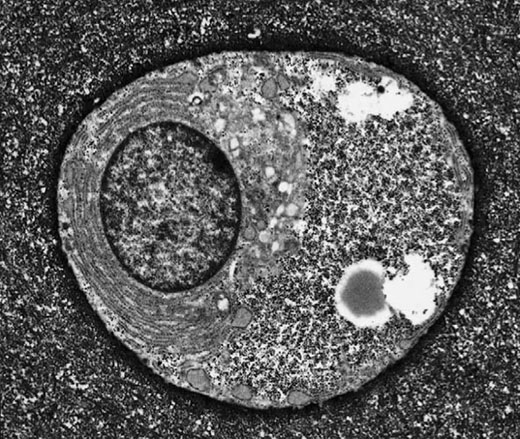
Electron micrograph of a typical articular chondrocyte (Archer & Francis-West, 2003).
Chondrocytes seem to be at the crux of our story because they are the single cell type found in cartilage. They are extremely active cells capable of responding to a range of mechanical and chemical stimuli. Research indicates that these responses are crucial to their creation and maintenance of viable cartilage, though the details of these processes are poorly understood.
In our study, we use computer models of the chondrocyte to better understand their response to their constantly changing environment. In particular, we look at how the cell’s resting membrane potential (RMP) evolves, as abnormal regulation of the RMP in these cells is linked to abnormal volume regulation when loaded, altered signaling and cell death. Focussing our attention on a single chondrocyte cell residing in deep regions of cartilage, we construct an ordinary differential equation (ODE) system model that links the membrane potential of the cell with the flux of various ions in and out of the cell membrane through ion channels. Our solver for this equation system is implemented in Octave, and a current version is available as Free Software under the GNU GPL.
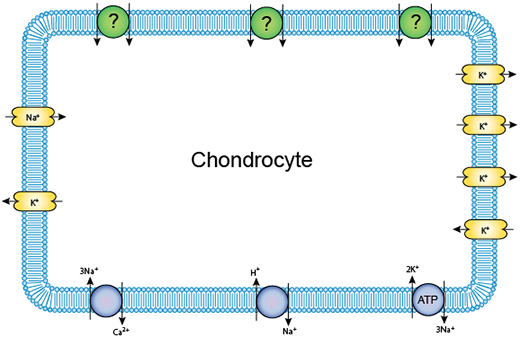
A schematic of the ion channels in a single chondrocyte.
When our computer model of the chondrocyte is subjected to several different clinically-relevant environments, we begin to gain an understanding of the underlying behavior of the cell. Our research hints at ideal conditions the cell must be subjected to as it relates to the synthesis of viable cartilage, and circumstances where this might be disrupted.
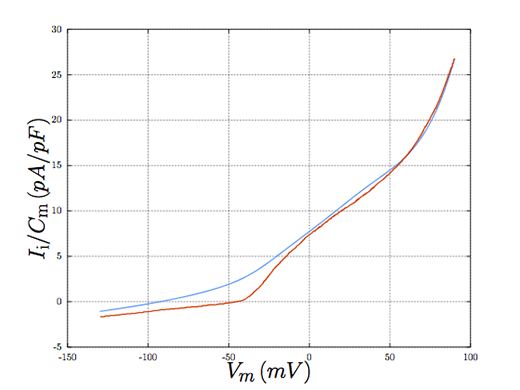
Comparing the model behaviour (blue) with experiment (red).
We envision that the insight gained from this work will help in multiple ways. First, it will help clinicians and biochemists design better drugs to treat the condition of osteoarthritis. Second, it will help suggest to patients subtle changes to their lifestyle which could help reduce the degradation of cartilage resulting from age, greatly improving their quality of life.